Phase I
*Cytochrome P450 looks for sites most electron rich and least sterically hindered
So aromatic rings with electron withdrawing groups (Halogens, Nitro, carboxylic acid etc..) are not oxidized.
So aromatic rings with electron withdrawing groups (Halogens, Nitro, carboxylic acid etc..) are not oxidized.
*The same drug can be oxidized at different sites.
so when looking at a drug we look for
(R is the rest of the molecule)
(R is the rest of the molecule)
1-Aromatic (benzene ring) → para hydroxylation
 2-Olifin (double bond) → diol
2-Olifin (double bond) → diol
 2-Olifin (double bond) → diol
2-Olifin (double bond) → diol
3- Methyl group attached to benzene (benzylic carbon) → alcohol
 4-Methyl group attached to C=C (allylic carbon) → alcohol
4-Methyl group attached to C=C (allylic carbon) → alcohol
 5-Carbon next to carbonyl and imin (benzodiazepine) → secondary alcohol
5-Carbon next to carbonyl and imin (benzodiazepine) → secondary alcohol
 6-long chain → ω (terminal) carbon or ω -1 (carbon before the terminal) → alcohol
6-long chain → ω (terminal) carbon or ω -1 (carbon before the terminal) → alcohol
 7-Alicyclic (saturated ring) → alcohol on para position.
7-Alicyclic (saturated ring) → alcohol on para position.

 4-Methyl group attached to C=C (allylic carbon) → alcohol
4-Methyl group attached to C=C (allylic carbon) → alcohol 5-Carbon next to carbonyl and imin (benzodiazepine) → secondary alcohol
5-Carbon next to carbonyl and imin (benzodiazepine) → secondary alcohol 6-long chain → ω (terminal) carbon or ω -1 (carbon before the terminal) → alcohol
6-long chain → ω (terminal) carbon or ω -1 (carbon before the terminal) → alcohol 7-Alicyclic (saturated ring) → alcohol on para position.
7-Alicyclic (saturated ring) → alcohol on para position.
8-Tertiary or secondary aliphatic amine → removal of a methyl group to give amine
.....(make sure to remove the smaller group)
.....(called oxidative dealkylation)
.....(make sure the alkyl group removed has H on it ).....(if the other group is small will be removed too

9-Tertiary nitrogen (nitrogen with no hydrogen on it) → N-oxide.

10-Primary amine (R-CH2-NH2) → NH3 (amonia) + R-CHO (carbonyl)

10-Primary amine (R-CH2-NH2) → NH3 (amonia) + R-CHO (carbonyl)
.......(oxidative deamination)
........(make sure the carbon next to N has H on it)
or
........ NH2 → NO2 (nitro). 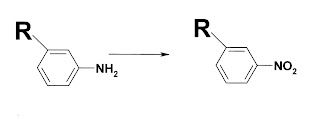 11-An OCH3 group → OH +OCHO
11-An OCH3 group → OH +OCHO
 11-An OCH3 group → OH +OCHO
11-An OCH3 group → OH +OCHO12-An (R-S-CH3) → removal of CH3 → R-SH + HCHO
..........the carbon removed should have H
or
..............→ Oxidation of the sulfur → Sulfoxide or sulfone.


13-A Sulfur attached to a carbon with double bond C=S → C=O (oxidative desufuration)
14- R-CH2OH (alcohol) → R-CHO (aldehyde) → R-CH2CH2CH2COOH (acid)
15- R-COOCH2R' (ester) → RCOOH (acid)+ R'-CH2OH (alcohol)
16-R-HNCO-R' (amide) → R-NH2 (amine) + R-'COOH (acid)
 *all the functional groups produced above (aromatic OH ; NH2 ; C=O ; COOH ; SO2 ; NO2 ; SH etc...) will be conjugated in phase II.
*all the functional groups produced above (aromatic OH ; NH2 ; C=O ; COOH ; SO2 ; NO2 ; SH etc...) will be conjugated in phase II.*If a drug has a functional group before metbolism e.g. OH or COOH or aromatic NH2 etc.. It can go directly to phase II and conjugates this functional group to a conjugating agent (mostly glucoronic acid)
*Aromatic amines are famous for conjugation to aAcetyl group.
Please apply all these rules to the drugs given in the lectures.
Good Luck

No comments:
Post a Comment